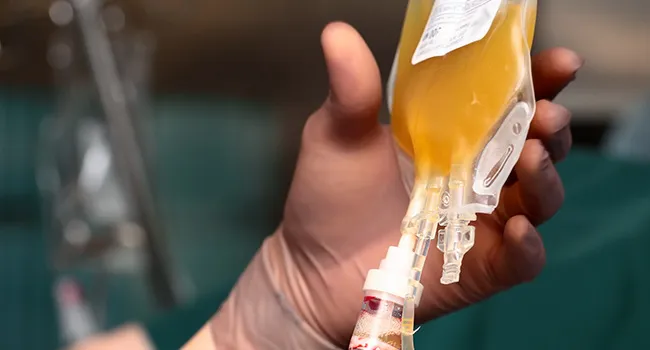
While the FDA did not name Ambrosia its release, it cautioned consumers that there is “no proven clinical benefit” to the transfusions. Although
from WebMD Health https://ift.tt/2SQhm6C

While the FDA did not name Ambrosia its release, it cautioned consumers that there is “no proven clinical benefit” to the transfusions. Although
0 comments:
Post a Comment